If you follow science, or science fiction, to any degree, great or small, you’ve probably heard the term “quantum entanglement” before. You may also have heard it referred to as “spooky action at a distance,” and understand that it somehow involves a weird connection between separated quantum particles that can “communicate,” in a sense, over long distances instantaneously. You may have read that quantum entanglement is a key aspect in proposed technologies that could transform society, namely quantum cryptography and quantum computing.
But it is difficult for a non-physicist to learn more about quantum entanglement than this, because even understanding it in a non-technical sense requires a reasonably thorough knowledge of how quantum mechanics works.
In writing my recently-published textbook on Singular Optics, however, I had to write a summary of the relevant physics for a chapter on the quantum aspects of optical vortices. I realized that, with some modification, this summary could serve as an outline for a series of non-technical blog posts on the subject; so here we are!
It will take a bit of work to really get at the heart of the problem; in this first post, I attempt to outline the early history of quantum physics, which will be necessary to understand what quantum entanglement is, why it is important, and why it has caused so much mischief for nearly 100 years!
Small disclaimer: though I am a physicist, I am not an expert on the weirder aspects of quantum physics, which have many pitfalls in understanding for the unwary! There is the possibility that I may flub some of the subtle parts of the explanation. This post is, in fact, an exercise for me to test my understanding and ability to explain things. I will revise anything that I find is horribly wrong.
Near the end of the 19th century, there was a somewhat broad perception that the science of physics was complete; that is, there were no more important discoveries to be made. This is encapsulated perfectly in an 1894 statement by Albert Michelson, “… it seems probable that most of the grand underlying principles have been firmly established … An eminent physicist remarked that the future truths of physical science are to be looked for in the sixth place of decimals.”
By 1900, the universe seemed to be well-described as a duality. Matter consisted of discrete particles (atoms), whose motion could be described by Newton’s laws of motion and law of gravitation, and light consisted of waves, whose evolution could be described by Maxwell’s equations for electromagnetism. In short: matter was made of particles, light was made of waves, and that covered everything that we observed. We will, in shorthand, call this “classical physics” going forward.
But there were still a number of mysteries that were perplexing and unsolved at the time. One mystery was the nature of atoms: atoms clearly had some sort of structure, because they absorbed and emitted light at isolated frequencies (colors), but what was that structure? There was much speculation in the early years of the 20th century related to this.
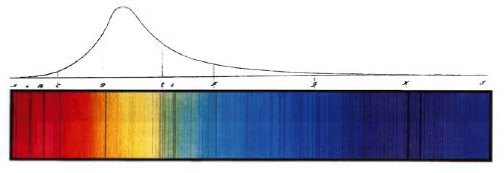
Fraunhofer’s 1814 drawing of the spectrum of sunlight. The dark lines in the lower color image aren’t mistakes; they’re discrete colors of light that are absorbed by atoms at the sun’s surface.
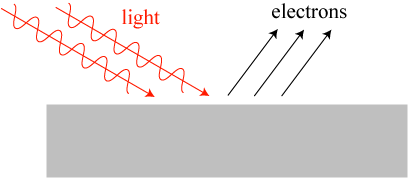
Another unsolved mystery was the origin of the phenomenon known as the photoelectric effect. In short: when light shines onto a metal surface under the right conditions, it can kick off electrons, as illustrated crudely below.
However, the photoelectric effect didn’t seem to work as classical physics predicted it would. The energy of electrons being kicked off of the metal didn’t increase with the brightness of the light beam, as one would expect from the classical theory; it increased with the frequency of light. If the light was below a certain frequency, no electrons at all would be kicked off. The brightness of the light beam only increased the number of electrons ejected.
The puzzle was solved by none other than Albert Einstein. In a 1905 paper, he argued that the photoelectric effect could be explained if light not only behaved as a wave but also as a stream of particles, later dubbed photons, each of which has an energy proportional to frequency. Higher frequency photons therefore transfer more energy to the ejected electrons. Also, a brighter light beam has more photons in it, resulting in more electrons getting ejected.
This was the first illustration of the concept of wave-particle duality: the idea that light has a dual nature as a wave and a stream of particles. Depending on the circumstances, sometimes the wave properties are dominant, sometimes the particle properties are; sometimes, both must be taken into account.
Einstein’s argument was a profound one, and answered other questions that had been troubling physicists for a number of years. For instance, the shape of the upper curve in Fraunhofer’s spectrum above, which shows the relative brightness of the different colors of sunlight, is known as a blackbody spectrum. It can be shown that the shape of the curve arises from the particle nature of light. Einstein won the 1921 Nobel Prize in Physics for his work on the photoelectric effect, which provided clear evidence that there was still more to understand about the fundamentals of physics.
So light, which was long thought to only be a wave, turns out to also be a particle! One might naturally wonder if the reverse is true: that matter, long thought to consist of particles, might also have wave properties? This was the idea that occurred to French physicist and PhD candidate Louis de Broglie in the early 1920s. As he would later say in his 1929 Nobel Lecture,
I thus arrived at the following overall concept which guided my studies: for both matter and radiations, light in particular, it is necessary to introduce the corpuscle concept and the wave concept at the same time. In other words the existence of corpuscles accompanied by waves has to be assumed in all cases.
Louis de Broglie put forth this hypothesis in his 1924 PhD dissertation, and though his work was considered radical at the time, the wave nature of electrons was demonstrated in 1927 in what is now known as the Davisson-Germer experiment.
The idea that electrons have wave properties resolved other physics mysteries. Remember the question about the structure of the atom? The first major piece of the puzzle to be found was the experimental discovery of the atomic nucleus in 1910 by Ernest Rutherford and his colleagues. It naturally followed that electrons must orbit the atomic nucleus, much like planets orbit the sun, but this still did not explain why atoms would only absorb and emit light at distinct frequencies.
In 1914, Danish physicist Niels Bohr solved the problem by introducing new physics. In the Bohr model of the atom, electrons are only allowed to orbit the nucleus with discrete values of orbital angular momentum, and could only release or absorb light by “jumping” between these discrete orbits. The orbits are labeled by an integer index n, as illustrated below. Bohr’s model reproduced exactly the emission and absorption spectrum of hydrogen, and was viewed as a major step in understanding atomic structure.
But why would electrons only orbit with those discrete values of angular momentum? This was a question that the physics of the time could not answer, and was in essence an unexplained assumption in Bohr’s model.
It so happened that de Broglie’s hypothesis, that electrons have wave properties, provided the explanation! de Broglie realized that, if the electron acted like a wave, then those waves could only “fit” around the nucleus when an integer number of wavelengths fit in an orbit. A rough illustration of this is below.

Visualization of de Broglie waves around an atom. Each more distant electron orbit has one extra “hump” in the electron wave.
Louis de Broglie was actually inspired by a very mundane example: a vibrating string! As he recalled in his Nobel lecture,
On the other hand the determination of the stable motions of the electrons in the atom involves whole numbers, and so far the only phenomena in which whole numbers were involved in physics were those of interference and of eigenvibrations. That suggested the idea to me that electrons themselves could not be represented as simple corpuscles either, but that a periodicity had also to be assigned to them too.
This is an experiment you can try at home with a string or a phone cord! Though you can shake a string at any frequency you want, there are only certain special isolated frequencies that will feel natural, known as resonance frequencies.

First few resonance modes of a string with fixed ends. Each mode has one more “hump” than the previous one.
So, by 1924, physicists were aware that both matter and light possess a dual nature as waves and particles. However, the situation between matter and light was not entirely equal. Since James Clerk Maxwell’s work in the 1860s, physicists had a set of equations, known as Maxwell’s equations, that could be used to describe how a light wave evolves in space and time. But nobody had yet derived an equation or set of equations that could describe how the wave associated with matter evolves.
This was a challenge undertaken by Austrian physicist Erwin Schrödinger in 1925, soon after de Broglie had suggested matter has wave properties. Within a year, using very clever arguments and intuition, Schrödinger derived an equation that accurately modeled the wave properties of electrons, now known as the Schrödinger equation. With the Schrödinger equation, it became possible to quantitatively model the behavior of electrons in an atom and accurately predict how they would absorb and emit light.
So, by 1927, the new quantum theory of light and matter was in reasonably good shape. There was experimental evidence of both the particle and wave nature of both light and matter, and the particle nature of light and the wave nature of matter had been experimentally confirmed. This is summarized in the table below, for convenience.
But there was one big puzzle on the matter side, as illustrated in the lower right corner: what, exactly, is “waving”? In other words, what does the wave part of a matter wave represent? In water waves, it is the water “waving” up and down, and conveying energy through this motion. In sound waves, it is the molecules of the air “waving” forward and back. In light waves, it is the electric and magnetic fields that are “waving.” But there was no obvious way to interpret what was doing the waving in a matter wave.
It turns out that the initial answer to the question, which would be formulated right around the years 1926-1927, would lead to some very strange philosophical implications of the new quantum theory. This will be discussed in part 2 of this series of posts!




I need part 2
I am going to share this with my 10 year old Grandson…..He will get it. Been looking for “Quantum Physics for Kids ” or QP for Dummies.
Doug Cook
619.302.2825
Cliff hanger!
Good job. I hope second part will come soon.
This post is awesome in every single aspect. Can’t wait for part 2!
Brilliant! So accessible, thank you.
Good so far. When is the next post?
When is the next post?
Hopefully within a couple of days!
Great post! looking forward to Part 2 🙂
Nice. A partical on Tuesday and a wave on Thursday.
Awesome post! In the fourth figure (de Broglie waves), you say higher modes correspond to larger distances from the atom. What is preventing higher modes from existing on every orbital level? For example, couldn’t you “fit” three, four, five, etc. humps on the n=2 circle?
The answer, I believe, is a mix of classical and quantum. A larger number of humps = a shorter wavelength = more momentum. A higher momentum particle in the same orbit, however, will not be in a stable orbit.
And we breathe it.
Very good explanations.
Reblogged this on Transcendence and commented:
For the QM freaks, here’s something awesome…
It is extremely difficult for three dimensional beings that see two dimensionally to understand fourth, fifth, etc. dimensional cubes or “masses.” This is a good first step for people who are interested in exercising their perception of our Universe. I remind people, constantly; not that long ago (considering the age of our planet) it was a “fact” that the Earth was flat and if you sailed in one direction…you would fall off the edge of the Earth. We now know that the Earth is not flat, but how long will it take for the majority to understand that it is not only three dimensional, but also…simultaneously…fourth, fifth, etc dimensional?
I salute you with Thanks ! You have shared your rare gift to put across the interconnectedness of complex into simple clear coherent that a layperson can understand and say ‘ Now I can see ‘ and ‘ Now I Understand ‘
You have inadvertently forgotten the Father of it all — Max Plank. He deserves at least a footnote!
Terrific overview!