Last week heralded the long-awaited arrival of a package I had ordered, the content of which seems rather unimpressive at first glance. It consists of a small metal cylinder, with an adjustable lens on one end and a screw on the other:
If you look into the lens of this device, called a “spinthariscope”, under most circumstances, you’ll almost certainly see nothing at all. With that in mind, you might be surprised to learn that such humble devices were in fact hugely popular in the early 1900s, being carried both as toys by children and as status symbols by the learned elite!
The secret of the spinthariscope’s success comes from the fact that it allows the seemingly impossible — the ability to watch individual radioactive decays happens with the naked eye!
So what is a spinthariscope? In essence, it is a self-contained radiation source and detector, and has elements as shown below:
A small radioactive source (the details of which we will discuss later) emits alpha particles that collide with a zinc sulfide (ZnS) screen. This screen gives off flashes of light (called scintillations) at the places the alpha particles hit. These minute flashes are magnified by a simple lens and can be viewed through the eyepiece. Every flash the viewer sees is the trace of a single atomic nuclear decay. By adjusting the bottom screw, one can effectively increase or decrease the rate at which alpha particles hit the screen, transforming a flood of particles into a trickle, or vice versa.
This is a pretty neat effect, and is worth blogging about in itself, but the spinthariscope also has historical significance: it was the first device invented that is able to detect individual radioactive particles, a precursor to the Geiger counter!
I’ve talked about the history and physics of radioactivity a number of times on this blog; see, for instance, here and here. A short history of the discovery of radioactivity will be helpful here. In 1896, radioactivity was serendipitously discovered by Paris researcher Henri Bequerel in the course of his investigations into fluorescence and phosphorescence. Inspired by the recent and sensational discovery of X-rays in 1895 by Wilhelm Röntgen, Becquerel wondered if “glow in the dark” materials might also give off X-rays. He wrapped photographic plates in black paper to protect them from sunlight, then placed a sample of uranium on the plate in the light. Becquerel expected the uranium to fluoresce X-rays, which would then darken the photographic plate, and he seemingly found this to be true. However, when he put the plates and the uranium sample in a dark drawer for a few days, the plates turned out to be developed even darker than before! Evidently some new mysterious emissions from the uranium were darkening the plate, and these emissions were quickly referred to as Becquerel rays.
This monumental discovery was overshadowed by the craze over X-rays for a few years, but this changed when Marie Curie began to discover radioactivity in other elements. She discovered radioactivity in thorium, but was scooped by several weeks in publication. However, in 1898 she and her husband Pierre isolated the new radioactive element polonium, and after several years of arduous labor the pair isolated a new and very powerful element, radium.
Radium ignited real excitement in the scientific community. It is more than a million times more radioactive than uranium, and seemed at the time to be a limitless energy source. The mystery captured the attention of a number of researchers, among them London chemist and physicist William Crookes (1832-1919).
 Crookes was already a well-established and successful scientist by the early 1900s. Around 1870 he invented the Crookes tube, an early electrical discharge tube that was instrumental in the discovery of both the electron and X-rays. Crookes’ specialty, however, was spectroscopy, and by measuring the light emission of atoms he discovered the element thallium in 1861, and helped identify the first isolated sample of helium on Earth in 1895. On a less distinguished note, Crookes was an avid investigator of spiritual phenomena, and his credulous nature so irked the scientific establishment that there was talk of depriving him of his Fellow status in the Royal Society.
Crookes was already a well-established and successful scientist by the early 1900s. Around 1870 he invented the Crookes tube, an early electrical discharge tube that was instrumental in the discovery of both the electron and X-rays. Crookes’ specialty, however, was spectroscopy, and by measuring the light emission of atoms he discovered the element thallium in 1861, and helped identify the first isolated sample of helium on Earth in 1895. On a less distinguished note, Crookes was an avid investigator of spiritual phenomena, and his credulous nature so irked the scientific establishment that there was talk of depriving him of his Fellow status in the Royal Society.
In 1903 Crookes joined in the enthusiastic investigation of the properties of radium. He published many of his results in the journal The Chemical News, perhaps not so difficult considering he was the editor!
The discovery that would lead to the spinthariscope was curiously accidental, just like the discovery of X-rays and radioactivity before. Crookes gave an account of it in an April 3, 1903 article entitled, “The emanations of radium”.* His description is somewhat dryer than the later popularized accounts. He begins by explaining his experiments involving radium and its interactions with radiation sensitive materials:
A solution of almost pure radium nitrate which had been used for spectrographic work, was evaporated to dryness in a dish, and the crystalline residue examined in a dark room. It was feebly luminous.
A screen of platinocyanide of barium brought near the residue glowed with a green light, the intensity varying with the distance separating them. The phosphorescence disappeared as soon as the screen was removed from the influence of the radium.
A screen of Sidot’s hexagonal blende (zinc sulphide), said to be useful for detecting polonium reactions, was almost as luminous as the platinocyanide screen in presence of radium, but there was more residual phosphorescence, lasting from a few minutes to half an hour or more according to the strength and duration of the initial excitement.
It is to be noted that the only thing observable to Crookes in his initial experiments was a continuous glow. He continues by discussing some of the physical effects of the radioactivity:
The persistence of radio-activity on glass vessels which have contained radium is remarkable. Filters, beakers, and dishes used in the laboratory for operations with radium, after having been washing in the usual way, remain radio-active; a piece of blende screen held inside the beaker or other vessel immediately glowing with the presence of radium.
The blende screen is sensitive to mechanical shocks. A tap with the tip of a pen-knife will produce a sudden spark of light, and a scratch with the blade will show itself as an evanescent luminous line.
A diamond crystal brought near the radium nitrate glowed with a pale bluish-green light, as it would in a “Radiant Matter” tube under the influence of cathodic bombardment. On removing the diamond from the radium it ceased to glow, but, when laid on the sensitive screen, it produced phosphorescence beneath which lasted some minutes.
A “radiant matter tube” is simply another name for a Crookes-type tube, which produces a glowing bluish-green beam of electrons. Crookes notes that the diamond glows with a similar light, but draws no immediate conclusions from this.
It was with these diamond manipulations that a slip-up led to the remarkable discovery:
During these manipulations the diamond accidentally touched the radium nitrate in the dish, and thus a few imperceptible grains of the radium salt got on to the zinc sulphide screen. The surface was immediately dotted about with brilliant specks of green light, some being a millimetre or more across, although the inducing particles were too small to be detected on the white screen when examined by daylight.
In popular descriptions, it is usually related that Crookes, having spilled the radium on the surface, used a magnifying glass to seek out the precious specks of radium. This might seem stingy to modern readers, but radium was one of the most precious substances in the world at that point. A paper by W.J. Hammer in the January 1903 issue of The Chemical News relates:
An extensive dealer in chemicals informed me recently that the treatment of 5000 tons of uranium residues would probably not result in the production of a kilo. of radium. The present market price in this country is 4.50 dollars per grm., or approximatelly 2000 dollars a pound.
And that’s in 1903 dollars! Crookes’ paper continues with a description of the phenomenon under magnification:
In a dark room, under a microscope with a 2/3-inch objective, each luminous spot is seen to have a dull centre surrounded by a luminous halo extending for some distance around. The dark centre itself appears to shoot out light at intervals in different directions. Outside the halo the dark surface of the screen scintillates with sparks of light. No two flashes succeed one another on the same spot, but are scattered over the surface, coming and going instantaneously, no movement of translation being seen.
Crookes establishes all of the basic properties that would form the spinthariscope in this first paper, even waxing somewhat poetic in his description:
A solid piece of radium nitrate is slowly brought near the screen. The general phosphorescence of the screen as visible to the naked eye varies according to the distance of the radium from it. On now examining the surface with the pocket lens, the radium being far off and the screen faintly luminous, the scintillating spots are sparsely scattered over the surface. On bringing the radium nearer the screen the scintillations become more numerous and brighter, until when close together the flashes follow each other so quickly that the surface looks like a turbulent luminous sea. When the scintillating points are few there is no residual phosphorescence to be seen, and the sparks succeeding each other appear like stars on a black sky.
It is worth noting that the significance of the discovery was the visualization of the particles, not the realization that radiation consists of particles. At the end of his paper, Crookes himself speculates on the nature of the radiation:
It seems probable that in these phenomena we are actually witnessing the bombardment of the screen by the electrons hurled off by radium with a velocity of the order of that of light; each scintillation rendering visible the impact of an electron on the screen.
As we will note, Crookes was not quite correct!
He wasted little time in making further observations. Some of these were described only a month later, in the May issue of The Chemical News** in an article titled “Certain properties of the emanations of radium”. Even in this short time, researchers had realized that the radioactivity of radium is much more complicated than initially assumed:
The emanations from radium are of three kinds. One set is the same as the cathode stream, now identified with free electrons — atoms of electricity projected into space apart from gross matter– identicla with “matter in the fourth or ultra-gaseous state,” Kelvin’s “satellites,” Thomson’s “corpuscles” or “particles”; disembodied ionic charges, retaining individuality and identity.
Electrons are deviable in a magnetic field. They are shot from radium with a velocity of about two-thirds that of light, but are gradually obstructed by collisions with air atoms.
Another set of emanations from radium are not affected by an ordinarily powerful magnetic field, and are incapable of passing through very thin material obstructions. They have about one thousand times the energy of that radiated by the deflectable emanations. They render air a conductor and act strongly on a photographic plate. These are the positively electrified atoms. Their mass is enormous in comparison with that of the electrons.
A third kind of emanation is also produced by radium. Besides the highly penetrating rays which are deflected by a magnet, there are other very penetrating rays which are not at all affected by magnetism. These always accompany the other emanations, and are Rontgen rays — ether vibrations — produces as secondary phenomena by the sudden arrest of velocity of the electrons by solid matter, producing a series of Stokesian “pulses” or explosive ether waves shot into space.
This is an excellent time to talk about what we now know about radioactivity and the nature of radium! The nuclei of all atoms consist of collections of positively-charged protons and electrically-neutral neutrons. Though the lighter elements, such as helium (2 protons, 2 neutrons) and oxygen (8 protons, 8 neutrons) are stable, the heavier elements are unstable and tend to fracture into pieces. The instability can be attributed to an excess of energy: the total energy of an element is more than the energy of the individual parts, making the nucleus “want” to break up.
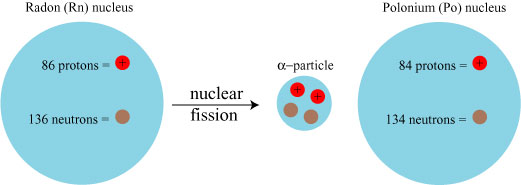
There are three methods by which a heavy atom can shed this excess energy; these processes are known as “alpha”, “beta” and “gamma” decay, the mysterious names a reflection of the historical fact that nobody knew what they were! Alpha decay is the release of an alpha particle (aka a helium nucleus: 2 protons, 2 neutrons) from the radioactive nucleus, as is the case in the decay of radium:

Radium decays into radon, which in turn decays by alpha decay into polonium:
Polonium in turn decays into a radioactive form of lead, and that lead decays via beta decay — the release of a high-velocity electron, turning a neutron into a proton:
The third type of radioactive decay is the release of an X-ray, though before it was identified as such it was known as a gamma ray. Because radium follows a complicated decay chain, all three types of radioactive emissions can be seen in a sample of radium to some degree.
This deep an understanding of the nucleus and radioactivity would come much later, however; the nucleus alone would not be discovered until 1909, in the famous Geiger-Marsden experiment.
Though Crookes could not understand the full details of what he was seeing, he still saw an opportunity in the “turbulent sea” of decays. The end of his May paper describes a new invention — the spinthariscope:
The Spinthariscope
A convenient way to show these scintillations is to fit the blende screen at the end of a brass tube with a speck of radium salt in front of it and about a millimetre off, and to have a lens at the other end. Focussing, which must be accurately effected to see the best effects, is done by drawing the lens tube in or out. I propose to call this little instrument the “Spinthariscope,” from the Greek word
, a scintillation.
 The spinthariscope, and all of Crookes’ radium experiments, debuted in person at a fancy party of the Royal Society on May 15th of 1903. This party, a “conversazione“, was held at the historic Burlington House in London and included hands on exhibits of all sorts of intriguing phenomena from all of the natural sciences. A detailed description of the party was provided in a 1903 issue of The Pharmaceutical Journal***, and lists, among other wonders, “five specimens of Hydrophidæ, the poisonous sea snakes which swarm round the coasts of India”, “the new coherer, as applied to wireless telegraphy, shown by Sir Oliver Lodge and Dr. Alexander Muirhead”, and “Rowland’s apparatus for the disintegration of micro-organisms by mechanical crushing when frozen by means of liquid air”. The real attention-grabber, however, was Crookes’ work:
The spinthariscope, and all of Crookes’ radium experiments, debuted in person at a fancy party of the Royal Society on May 15th of 1903. This party, a “conversazione“, was held at the historic Burlington House in London and included hands on exhibits of all sorts of intriguing phenomena from all of the natural sciences. A detailed description of the party was provided in a 1903 issue of The Pharmaceutical Journal***, and lists, among other wonders, “five specimens of Hydrophidæ, the poisonous sea snakes which swarm round the coasts of India”, “the new coherer, as applied to wireless telegraphy, shown by Sir Oliver Lodge and Dr. Alexander Muirhead”, and “Rowland’s apparatus for the disintegration of micro-organisms by mechanical crushing when frozen by means of liquid air”. The real attention-grabber, however, was Crookes’ work:
Another exhibit which attracted more attention than anything else was that by Sir William Crookes, illustrative of the properties of the emanations of radium. There were autoradiographs, photographs of radium emanations, luminous effects of radium emanations, and an ingenious little instrument which Sir William Crookes calls a spinthariscope, intended as a convenient contrivance to show the scintillations of a piece of radium nitrate.
According to other accounts, the spinthariscope was quite a sensation, being sold both as toys and as serious scientific instruments! It didn’t take very long for the ‘scope to be commercialized, as this 1903 ad that appeared in Nature indicates:

In hindsight, radium was a very bad choice for a material to be disseminated amongst the general public, much less children. Radium itself is chemically similar to calcium, and can replace the calcium in bones if ingested, leading to cancer. Also, its decay product radon is a gas which also has significant risks of ingestion. The reality of radium’s danger finally came to a head in the 1920s, when a number of women who worked painting luminous radium watch dials fell seriously ill and died from radium poisoning. The story of the “radium girls” was recently told in a three part post by Deborah Blum (here, here and here). Nowadays, spinthariscopes contain thorium or americium, elements which present negligible risk.
With all this in mind, I wasn’t sure what to expect from my own spinthariscope. The scintillations from it are so faint that one must sit in the dark for 20 minutes to allow one’s eyes to become sensitive enough to see them. The flyer that came with the device suggested that some people are born without enough light sensitivity and can never see the flashes. This statement made me wonder whether I’d been the victim of an elaborate con: “Oh, you don’t see anything? Well, you’re one of the unlucky ones.”
As I sat alone in the powder room, musing over whether there was actually radioactive material in my spinthariscope, I began to notice a dim glow emanating from the eyepiece. I gave it a few more minutes, and looked inside. There it was, Crookes’ “luminous turbulent sea”. I spent quite a few minutes sitting there, looking at the flashes of light wash over the screen like rain on a window, somewhat in awe of the depth of the history and physics that the simple spinthariscope represents.
**********************
* W. Crookes, “The emanations of radium,” Chemical News 87 (1903), 157-158.
** W. Crookes, “Certain properties of the emanations of radium,” Chemical News 87 (1903), 221.
*** “The Royal Society’s Conversazione,” The Pharmaceutical Journal 70 (1903), 714.
**** You can get your own spinthariscope from United Nuclear. I got the “Super Spinthariscope”, which has an adjustable eyepiece, though it likely isn’t essential. It’s really hard to focus on an ultra-dim light source that is rapidly flickering anyway!






During the late 1940’s a popular children’s radio show offered a ring that had polonium and some sort of screen. I bought the cereal or Ovalteen and received one and it worked just fine.
Very neat! It’s quite fascinating to see how public perception of radioactivity has swung from highly positive to highly negative. (Both views are improperly extreme, IMHO.)
Incidentally, is this the ring you’re referring to?
Great post! I’d love to see what the old toy versions looked like some day.
Thanks! There’s a picture of one of the toy versions on Wikipedia; I’ll have to look around for other models.
In space, you can see flashes without the aid of an external scintillant.
See the “light flash” diagram here
http://people.roma2.infn.it/~lazio/html/sb/
Very neat — and a little unnerving! Kind of a constant reminder that one is being bombarded with high energy radiation.
P.S. That will make an excellent Twitter #weirdscifacts for the day! 🙂
I remember playing with one when I was young. I think I got rid of if when I realized I was holding a radioactive source up to my eye for extended period of time as a toy.
Ha! Yes, it makes me a little nervous as well. As I understand it, though, because the source is an alpha emitter, there isn’t really any significant radiation escaping the device. (Nevertheless, I don’t walk around with it in my pocket or sleep with it under my pillow!)
fantastic! does it have a number to know how much it is dangerous?
There isn’t any radiation dosage listing on the device. I think the device actually has a negligible dosage and doesn’t require a rating. Otherwise, they couldn’t just sell it to any crazy person like me!
Awesome! Do you know about how long one of these would last before the sample is too decayed to see? I wasn’t sure which isotopes were used in the spinthariscopes, nor how much is in the sample.
Well the thorium in my spinthariscope has a 14.2 billion year half-life. From what the company says in its flyer, it should pretty much last at the same level of activity “forever”, or at least well after my life. I don’t know how much it contains, however.
Is this site active? I can see it was about a year ago! I cry for help with a couple of ‘phosphor’ queries.
My home made Spinthariscope (Smoke alarm’ Americium and TV screen scrapings for a screen) works OK in that I have a decent display of scintillations. HOWEVER, and here are my possibly stupid questions:-
1. I used the phosphor scrapings from a colour TV, should I not see coloured scintillations?
2. Is it feasible to make a spinthariscope screen which, divided into say three 120 degree segments each coated with the different phosphors so as to exhibit the three primary colours?
3. Imagining for a moment that I had the money, what would be the ‘chemical name’ of the three coatings required and if possible – where I should look to acquire them?
Many Thanks – Douglas Blackmore
From an old school TV repair man I heard that the colour TV phosphors are very poisonous (B&W probably too). They contain Rhodium Carbonate for instance, which will happily hand you leukaemia under unfavourable conditions.
On a spinthariscope unrelated note: A TV technician once shot himself through the heart while decommissioning an old CRT. He was bent over the CRT while breaking the glass nipple inside the ring of contacts connected to the electron gun inside, in order to let air flow into the tube. The glass nipple shot into the tube with the air and penetrated the screen and his heart.
Our retinas contain two types of cells: rods and cones. The rods are sensitive to low levels of light, but provide basically black and white vision. The 3 types of cones provide colour vision, but respond only to large levels of light (e.g. daytime). Astronomers looking through small telescopes at dim nebulae (e.g. the Orion Nebula) can see the glow, but not the red colour shown in magazine photographs. So probaly you wouldn’t see colour, even though you use colour phosphors.
My old Gilbert chemistry set had one – loved it!
Perhaps a factor in my becoming a chemist.
If you put the dial from an old luminous WWII aircraft meter or from an early 20th century luminous watch under a microscope, after dark adaptation you can see the individual atomic disintegrations in the radium paint.
Pingback: Quora
Hi,
I read: “The third type of radioactive decay is the release of an X-ray, though before it was identified as such it was known as a gamma ray.”
Should that not be the other way around?
I just received my Super Spinthariscope from United Nuclear yesterday, and had a fun time experimenting with it at night. And yes, I tucked it under my pillow ;-).
I had difficulty sharpening the image though, so I’m considering putting a very tiny black dot on the screen so I can adjust the lens during daylight.
A very cool educational gadget to have!
They’re a lot of fun! I need to break mine out again.
Like George above, I got one in a Gilbert set — and still have it after 60 years.
I’ve often wondered about how long it would last. I thought that possibly the radioactive source (I’ve always thought it was radium; glad to know it’s not) would be exhausted after just a few years and would be very small, but it seems to be just about as strong as when it was when new. I guess a few billion years of half-life helps that.
BTW, it looks nothing like any of these in the pictures referenced. It’s similar in shape to a jeweler’s loupe pictured here: https://en.wikipedia.org/wiki/File:Jeweler's_Loupe.jpg
Sorry if this gets to the wrong place! I wanted to send the below as a Reply to a comment John had made in
your ““ Continue reading “The spinthariscope — see atoms decay before your eyes!” link which was in your email. Unfortunately REPLYING to his comment requires a URL which I don’t have! Can you help me? Thank you!
Dear John,
Sorry this question is about the COMMENT site and not a ‘scientific’ subject – Can you accommodate a site query?
Out of the blue I got two emails yesterday – They both had: – as Subject “[New Comment] The Spinthariscope see . . .” and they both had “Skulls in the Stars [comment-reply@wordpress.com]” as their FROM titles. They gave a clickable link “Continue reading “The spinthariscope — see atoms decay before your eyes!” which led to a very interesting ‘article’ including comments. Upon reading the comments I discovered I had contributed (asked for help) to the site back in 2012. But I can’t remember, from all those years ago, how I found the site, how to ‘join the site, or even the name and address of the site?
I am bamboozled by the FROM address (see above) in the emails – looks more like an email address than a website (Club? /Association?) address.
Can you orientate me? What is, How do I sign up to, this COMMENT/Wordpress ‘Association’?
Thank You – Douglas Blackmore
So amazing. I just purchsed mine from United Nuclear.